فهرست مطالب
Biolmpacts
Volume:12 Issue: 5, Sep 2022
- تاریخ انتشار: 1401/06/19
- تعداد عناوین: 9
-
-
Pages 395-404Introduction
The limited efficacy of BCG (bacillus Calmette–Guérin) urgently requires new effective vaccination approaches for the control of tuberculosis. Poly lactic-co-glycolic acid (PLGA) is a prevalent drug delivery system. However, the effect of PLGA-based nanoparticles (NPs) against tuberculosis for the induction of mucosal immune response is no fully elucidated. In this study, we hypothesized that intranasal immunization with culture filtrate protein-10 (CFP10)-loaded PLGA NPs (CFP10-NPs) could boost the protective immunity of BCG against Mycobacterium bovis in mice.
MethodsThe recombinant protein CFP10 was encapsulated with PLGA NPs to prepare CFP10-NPs by the classical water–oil-water solvent-evaporation method. Then, the immunoregulatory effects of CFP10-NPs on macrophages in vitro and on BCG-immunized mice in vivo were investigated.
ResultsWe used spherical CFP10-NPs with a negatively charged surface (zeta-potential −28.5 ± 1.7 mV) having a particle size of 281.7 ± 28.5 nm in diameter. Notably, CFP10-NPs significantly enhanced the secretion of tumor necrosis factor α (TNF-α) and interleukin (IL)-1β in J774A.1 macrophages. Moreover, mucosal immunization with CFP10-NPs significantly increased TNF-α and IL-1β production in serum, and immunoglobulin A (IgA) secretion in bronchoalveolar lavage fluid (BALF), and promoted the secretion of CFP10-specific interferon-γ (IFN-γ) in splenocytes of mice. Furthermore, CFP10-NPs immunization significantly reduced the inflammatory area and bacterial load in lung tissues at 3-week post-M. bovis challenge.
ConclusionCFP10-NPs markedly improve the immunogenicity and protective efficacy of BCG. Our findings explore the potential of the airway mucosal vaccine based on PLGA NPs as a vehicle for targeted lung delivery.
Keywords: PLGA, Nanoparticles, CFP10, Mycobacterium bovis, Mucosal immunization -
Pages 405-414Introduction
Hypoxia context is highly specific for tumors and represents a unique niche which is not found elsewhere in the body. Clostridium novyi is an obligate anaerobic bacterium. It has a potential to treat tumors. The aim of this study was to produce the C. novyi nontoxic spores and to investigate its oncolytic effect on breast cancer in mice model.
MethodsPrimarily, the lethal toxin gene in C. novyi type B was removed. Colonies were isolated using PCR testing. To assure the removal of alpha-toxin, plasmid extraction and in vivo assay were conducted. Next, to treat breast cancer model in different sizes of tumors, a single dose of spores of C. novyi nontoxic was tested.
ResultsThe results denoted that C. novyi nontoxic lost lethal toxin and a ppeared to be safe. For smaller than 1000 mm3 tumors, a single dose of C. novyi nontoxic was able to cure 100% of mice bearing breast tumors. Hence the mice remained free of tumor relapse. Tumors larger than 1000 mm3 were not cured by a single dose of C. novyi nontoxic treatment.
ConclusionThe experiment concluded that the C. novyi nontoxic might be a suitable and safe candidate, a novel therapeutic approach to encounter such hypoxic regions in the center of tumors. Research also showed that bacteriolytic therapy by C. novyi nontoxic could lead to regression in small tumor.
Keywords: Clostridium novyi, Nontoxic, Alpha toxin, Spore, Breast cancer, Hypoxia -
Pages 415-429Introduction
Malignant breast cancer (BC) frequently contains a rare population of cells called cancer stem cells which underlie tumor relapse and metastasis, and targeting these cells may improve treatment options and outcomes for patients with BC. The aim of the present study was to determine the effect of silibinin on the self-renewal capacity, tumorgenicity, and metastatic potential of mammospheres.
MethodsThe effect of silibinin on viability and proliferation of MCF-7, MDA-MB-231 mammospheres, and MDA-MB-468 cell aggregation was determined after 72-120 hours of treatment. Colony and sphere formation ability, and the expression of stemness, differentiation, and epithelial-mesenchymal-transition (EMT)-associated genes were assessed by reverse transcription-quantitative polymerase chain reaction (qRT-PCR) in mammospheres treated with an IC50 dose of silibinin. Additionally, the antitumor capacity of silibinin was assessed in vivo, in mice.
ResultsThe results of the present study showed that silibinin decreased the viability of all mammospheres derived from MCF-7, MDA-MB-231, and MDA-MB-468 cell aggregation in a dose-dependent manner. Colony and sphere-forming ability, as well as the expression of genes associated with EMT were reduced in mammospheres treated with silibinin. Additionally, the expression of genes associated with stemness and metastasis was also decreased and the expression of genes associated with differentiation were increased. Intra-tumoral injection of 2 mg/kg silibinin decreased tumor volumes in mice by 2.8 fold.
ConclusionThe present study demonstrated that silibinin may have exerted its anti-tumor effects in BC by targeting the BC stem cells, reducing the tumorgenicity and metastasis. Therefore, silibinin may be a potential adjuvant for treatment of BC.
Keywords: Breast cancer stem cells, Silibinin, Mammospheres, Epithelial to mesenchymal transition -
Pages 431-438Introduction
Acute kidney injury (AKI) may have a negative effect on mitochondrial hemostasis and bioenergetics as well as coenzyme Q10 (CoQ10) content. PGC-1α, AMPK, sirtuin 1 (Sirt1), and Sirt3, as the key metabolic regulators under nutritional stress, stimulate energy production via mitochondrial biogenesis during AKI. However, no report is available on the relationship between CoQ10 level and nutrient sensors in the pathophysiology of AKI caused by Hemiscorpius lepturus scorpion envenomation.
MethodsThree doses of venoms (1, 5, and 10 mg/kg) were administered by subcutaneous (SC) injection to male albino mice. The animals were sacrificed 1 day or 7 days after administration of venom and their kidneys were collected to analyze gene expression involved in AKI, nutrient sensors, and apoptosis signaling activation by real-time polymerase chain reaction (PCR) and the measurement of CoQ10 level using the High-performance liquid chromatography (HPLC) method.
ResultsThe data indicated a significant decrease in CoQ10 level after the administration of venom in 5 and 10 mg/kg. In addition, 1 day after the treatment, a significant over-expression of Sirt1 (5 and 10 mg/kg) was observed compared with normal mice. Overexpression of Sirt3 occurred 1 day and 7 days after treatment only at the dose of 5.0 mg/kg of venom. Furthermore, over-expression of AMPK as an important mitochondrial energetic sensor happened 1 day and 7 days after the injection of venom (5 mg/kg) (P<0.01). The significant increase in the gene expression of caspase-9 and 3 after the injection of venom (5 and 10 mg/kg) confirmed the role of cell death signaling.
ConclusionThe venom-induced energy-sensing pathways have a key role in gene expression of PGC-1α, AMPK, Sirt3, and CoQ10 content after venom-induced AKI.
Keywords: Acute kidney injury, Coenzyme Q10, Sirtuin 3, Sirtuin 1, 5' AMP-activated protein kinase, envenomation -
Pages 439-448Introduction
Inflammation is one of the most important mechanisms involved in cisplatin-induced acute kidney injury (AKI). Mesenchymal stromal/stem cells (MSCs) exhibit anti-inflammatory and immunomodulatory abilities. Human endometrial stromal/stem cells (hEnSCs) exhibit similar properties to MSCs. These cells secrete immunoregulators, so we investigated the inflammatory aspect of hEnSCs in the treatment of cisplatin-induced AKI in Wistar rats.
MethodsEach group consisted of 6 male Wistar rats. Groups were as follows: sham, model (5 mg/kg cisplatin, IP), and treatment (1 million hEnSCs, IV, 3 hours after cisplatin). Renal function, histopathology, proliferation rate, infiltration of CD3+ T cell, and expression of Il-10 and cystatin c (Cst3) were assessed on day 5. DiI-labeled cells were tracked in kidney and liver on days 4 and 14.
ResultsHEnSC transplantation improved cisplatin-induced injuries such as renal dysfunction and tissue damage. The highest levels of pathologic scores and hyaline cast formation were observed in the model group while hEnSCs transplantation resulted in their reduction (154.00 ± 14.95, 8.00 ± 1.41 vs. 119.40 ± 5.43, 2.50 ± 1.05). The percentage of Ki-67 positive cells in the treatment group increased while cisplatin decreased proliferation (39.91 ± 5.33 vs. 23.91 ± 3.57 in glomeruli and 39.07 ± 2.95 vs. 16.61 ± 3.25 in tubules). The expression of Cst3 and Il-10 was higher in the model and treatment groups, respectively. DiI-labeled cells were observed in the renal tubules and liver lobes on days 4 and 14.
ConclusionHEnSCs may ameliorate cisplatin-induced AKI through anti-inflammatory and immunomodulatory effects and/or through paracrine effects.
Keywords: Inflammation, Human endometrial stromal, stem cell, Rat, Cisplatin, Acute kidney injury -
Pages 449-461Introduction
Recently, MicroRNAs have gained increasing popularity as a novel nucleic acid-mediated medicine to regulate cancer-related protein expression. MicroRNA-21 (miR-21) is known as an oncogenic microRNA which is overexpressed in almost all cancers, including ovarian carcinoma that causes cisplatin (cis-Pt) resistance and vascular endothelial growth factor (VEGF) upregulation. So, miRNA-based therapy can be regarded as knocking down miR-21 expression, inducing tumor cell apoptosis, and suppressing tumor-associated angiogenesis.
MethodsPEG5k-carboxymethylated polyethyleneimine nanohydrogels (PEG5k-CMPEI) were loaded with AntagomiR-21 (As-21) at different ratios of nitrogen to phosphorus (N/P). Particle size and ζ potential were determined for the As-21 loaded nanohydrogels. In the cellular experiments, miR-21 expression, cytotoxicity, and cis-Pt sensitivity were studied on A2780 ovarian cancer cell lines. Finally, tumor cell apoptosis and tumor cell-associated angiogenesis were explored in vitro and in vivo.
ResultsThe nanohydrogels, featuring homogeneous size distribution and redox-responsiveness, were steadily loaded by As-21 at the optimum N/P ratio of 5 without any aggregation as determined by transmission electron microscopy (TEM). As-21-loaded nanohydrogels caused sequence-specific suppression of miR-21 expression and provoked apoptosis through ROS generation and caspase 3 activation. Cisplatin cytotoxicity was remarkably enhanced in A2780R as compared to A2780S following co-incubation with As-21-loaded nanohydrogels. Interestingly, the condition of the medium derived from As-21 nanohydrogel-treated A2780R cells inhibited VEGF suppression in human umbilical vein endothelial cells (HUVECs) and the formation of tubes in Matrigel. Moreover, the condition medium caused angiogenesis inhibition in the chicken chorioallantoic membrane (CAM) model.
ConclusionThese results suggest that nanohydrogel-based delivery of As-21 can be a promising neoadjuvant therapy for treating resistant tumors via apoptosis induction and angiogenesis suppression.
Keywords: Nanohydrogel, AntagomiR-21, MicroRNA Delivery, Apoptosis, Angiogenesis -
Pages 463-470Introduction
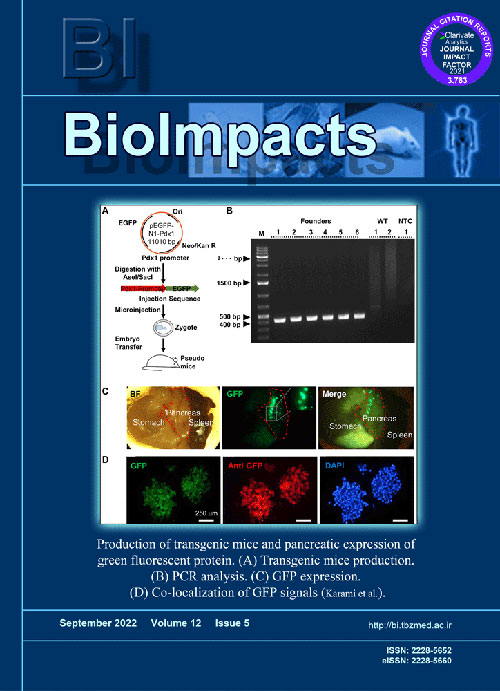
Measurement of pancreatic beta cell mass in animal models is a common assay in diabetes researches. Novel whole-organ clearance methods in conjunction with transgenic mouse models hold tremendous promise to improve beta cell mass measurement methods. Here, we proposed a refined method to estimate the beta cell mass using a new transgenic Tg(Pdx1-GFP) mouse model and a recently developed free-of-acrylamide clearing tissue (FACT) protocol.
MethodsFirst, we generated and evaluated a Tg(Pdx1-GFP) transgenic mouse model. Using the FACT protocol in our model, we could quantify the beta cell mass and alloxan-induced beta cell destruction in whole pancreas specimens.
ResultsCompiled fluorescent images of pancreas resulted in enhanced beta cell mass characterization in FACT-cleared sections (2928869±120215 AU) compared to No-FACT cleared sections (1292372±325632 AU). Additionally, the total number of detected islets with this method was significantly higher than the other clearance methods (155.7 and 109, respectively). Using this method, we showed green fluorescent protein (GFP) expression confined to beta cells in Tg(Pdx1-GFP) transgenic. This enhanced GFP expression enabled us to accurately measure beta cell loss in a beta cell destruction model. The results suggest that our proposed method can be used as a simple, and rapid assay for beta cell mass measurement in islet biology and diabetes studies.
ConclusionThe Tg(Pdx1-GFP) transgenic mouse in conjunction with the FACT protocol can enhance large-scale screening studies in the field of diabetes.
Keywords: Beta cell mass, Diabetes, Transgenic mouse, FACT protocol -
Pages 471-476Introduction
Poly(ethylene oxide) (PEO) is the most common polymer used in commercial abuse-deterrent tablets. Due to its vulnerability to high-temperature manipulation, we investigated abuse-deterrent capability and the toxicity of this polymer upon thermal treatments at 80°C and 180°C for 1 hour.
MethodsTablets (200 mg PEO and 300 mg Avicel®) were directly compressed under 2000 lb. The thermally manipulated PEOs were evaluated for their viscosity, crushability, structural changes, and cell toxicity.
ResultsOur findings showed that 180°C-treated tablets underwent some degrees of oxidative degradation with profound toxicity in both mesenchymal stem cells and MG63 cells. The 180°C-treated tablets exhibited almost no resistance against crushing and were prone to abuse. While thermal processing of PEO at around its melting temperature is a common approach to enhance crush resistance of its dosage forms, thermal manipulation at close to the PEO’s oxidation temperature can lead to structural changes, dramatic loss of crush and extraction resistance, and significant cell toxicity.
ConclusionSimilar to the low molecular weight PEO, when thermally manipulated at its thermo-oxidative temperature, the high molecular weight PEO loses its deterrence performance and causes severe cell toxicity.
Keywords: Abuse deterrent, Crush resistance, Extraction resistance, Thermal manipulation, Poly(ethylene oxide), Cytotoxicity -
Pages 477-478
Pregnancy and childbirth often threaten the life and health of the fetus. The greatest threat to the fetus during these periods is intrauterine hypoxia. The threat of intrauterine fetal hypoxia increases during natural childbirth and decreases during caesarean section. Therefore, it is no coincidence that the rate of C-section births is increasing worldwide. However, the generally accepted recommendations on the choice of caesarean delivery need to be clarified. A new test is needed to simulate intrauterine hypoxia and predict fetal survival during natural childbirth. Such a test would improve current C-section recommendations and newborn health outcomes. The most appropriate basis for such a functional test is the generally accepted Stange test. The fact is that the Stange test is a very easy to use and accurate functional test based on the duration of the longest breath hold. For more than 100 years, the Stange test has been successfully used to assess the adaptation reserves of adults to hypoxia in real time. The purpose of this letter is to present a new easy-to-use functional test designed to assess the resistance to hypoxia not only of the pregnant woman, but also of her fetus in real time. This new test could be a new vector in obstetric practice aimed at improving neonatal health and reducing infant mortality during delivery.
Keywords: Birth, Caesarean section, Hypoxia, Fetal resistance, Functional test