فهرست مطالب
Biolmpacts
Volume:12 Issue: 1, Jan 2022
- تاریخ انتشار: 1400/10/21
- تعداد عناوین: 9
-
-
Pages 3-7Introduction
Krabbe disease (KD) or globoid cell leukodystrophy (GLD) is one of the lysosomal disorders affecting central and peripheral nervous systems (CNS and PNS). It is caused by mutations on the galactocerebrosidase (GALC) gene. Affected individuals accumulate undegraded substrates and suffer from neuroinflammation.
MethodsHematopoietic stem cell transplantation (HSCT) has been partially successful in treating patients with KD when accomplished prior to the onset of symptoms. The success is credited to the ability of the hematopoietic stem cells in providing some GALC enzyme to the CNS and eradicating potential neuroinflammation. Combination of the HSCT with some other GALC-providing strategies has shown synergistic effects in the treatment of the mouse model of this disease.
ResultsHere, the possibility of eliminating HSCT in the treatment of human patients and replacing it with a single therapy that will provide sufficient GALC enzyme to the nervous systems is suggested. Such treatment, if started during the asymptomatic stage of the disease, not only may eradicate the enzyme deficiency, but may also keep any neuroinflammation at bay.
ConclusionSuccessful treatment of the KD may be possible by restoring consistent and sufficient GALC expression in CNS and PNS.
Keywords: Krabbe disease, GLD, GALC, Gene therapy, Bone marrow transplantation, BMT -
Pages 9-20Introduction
Drugs with no indication for the treatment of cardiovascular diseases (e.g., drugs employed to treat COVID-19) can increase the risk of arrhythmias. Of interest, a six-fold increase in the number of arrhythmic events was reported in patients with severe COVID-19. In this study, we reviewed (i) the pro-arrhythmic action of drugs given to patients with COVID-19 infection, and (ii) the effects of inflammatory cytokines on cardiac ion channels and possible generation of arrhythmias.
MethodsWe conducted a literature search on the drugs with purported or demonstrated efficacy against COVID-19 disease, emphasizing the mechanisms by which anti-COVID-19 drugs and inflammatory cytokines interfere with cardiac ion channels.
ResultsAntibiotics (azithromycin), antimalarials (hydroxychloroquine, chloroquine), antivirals (ritonavir/lopinavir, atazanavir), and some of the tyrosine kinase inhibitors (vandetanib) could induce long QT and increase risk for ventricular arrhythmias. The pro-arrhythmic action results from drug-induced inhibition of Kv11.1 (hERG) channels interfering with the repolarizing potassium IKr currents, leading to long QT and increased risk of triggered arrhythmias. At higher concentrations, these drugs may interfere with IKs, IK1, and/or Ito potassium currents, and even inhibit sodium (INa) and calcium (ICa) currents, inducing additional cardiac toxicity. Ibrutinib, an inhibitor of Bruton’s TK, increased the incidence of atrial fibrillation and ventricular tachycardia associated with a short QT interval. Inflammatory cytokines IL-6 and TNF-α inhibit IKr and Ito repolarizing potassium currents. High levels of inflammatory cytokines could contribute to the arrhythmic events. For remdesivir, favipiravir, dexamethasone, tocilizumab, anakinra, baricitinib, and monoclonal antibodies (bamlanivimab, etesevimab, and casirivimab), no evidence supports significant effects on cardiac ion channels, changes in the QT interval, and increased risk for ventricular arrhythmias.
ConclusionThis study supports the concept of hERG channel promiscuity. Different drug classes given to COVID-19 patients might delay repolarization, and increase the risk of ventricular arrhythmias. The presence of comorbid pro-arrhythmic disease states, and elevated levels of pro-arrhythmic cytokines, could increase the risk of ventricular arrhythmias. Discontinuation of nonessential drugs and correction of electrolyte abnormalities could prevent severe ventricular arrhythmias. Altogether, the most effective therapies against COVID-19 (remdesivir, dexamethasone, monoclonal antibodies) lack pro-arrhythmic activity.
Keywords: Drugs, COVID-19, Torsade de Pointes, Long QT, Antivirals, Cytokines, hERGpotassium channels, Tyrosine kinase inhibitors -
Pages 21-32Introduction
Recent studies showed that rapamycin, as a mammalian target of rapamycin (mTOR) inhibitor, could have beneficial therapeutic effects for the central nervous system (CNS) related diseases. However, the immunosuppressive effect of rapamycin as an adverse effect, the low water solubility, and the rapid in vivo degradation along with the blood-brain barrier-related challenges restricted the clinical use of this drug for brain diseases. To overcome these drawbacks, a transferrin (Tf) decorated nanostructured lipid carrier (NLC) containing rapamycin was designed and developed.
MethodsRapamycin-loaded cationic and bare NLCs were prepared using solvent diffusion and sonication method and well characterized. The optimum cationic NLCs were physically decorated with Tf. For in vitro study, the MTT assay and intracellular uptake of nanoparticles on U-87 MG glioblastoma cells were assessed. The animal biodistribution of nanoparticles was evaluated by fluorescent optical imaging. Finally, the in vivo effect of NLCs on the immune system was also studied.
ResultsSpherical NLCs with small particle sizes ranging from 120 to 150 nm and high entrapment efficiency of more than 90%, showed ≥80% cell viability. More importantly, Tf-decorated NLCs in comparison with bare NLCs, showed a significantly higher cellular uptake (97% vs 60%) after 2 hours incubation and further an appropriate brain accumulation with lower uptake in untargeted tissue in mice. Surprisingly, rapamycin-loaded NLCs exhibited no immunosuppressive effect.
ConclusionOur findings proposed that the designed Tf-decorated NLCs could be considered as a safe and efficient carrier for targeted brain delivery of rapamycin which may have an important value in the clinic for the treatment of neurological disorders.
Keywords: Rapamycin, Nanostructured lipid carrier, Transferrin, Brain delivery -
Pages 33-42Introduction
Aerobic vaginitis (AV) is a type of vaginal infection that occurs at the reproductive age of women. In this study, we aimed to study the possible anti-AV therapeutic effects of silver nanoparticles (AgNPs) and L-carnitine (LC) in the mouse model.
MethodsAV model was established by intra-vaginal inoculation of 108 CFU/mL Staphylococcus aureus and Escherichia coli (1:1) in adult NMRI mice. Susceptibilities of the bacteria were examined against AgNPs by inhibitory concentration (IC-50 and IC-90) and minimum biofilm inhibitory concentration (MBIC- 90) methods. The regimens therapy was intra-vaginal inoculation of AgNPs at MBIC- 90 and a daily injection of 250 mg/kg LC for two weeks. Mice were classified into healthy (control) and AV groups and then treated by LC, AgNPs, and AgNPs + LC. The vaginal smears were taken daily and tissue sections were prepared using the hematoxylin and eosin (H & E) method.
ResultsMinimum inhibitory concentrations (MICs) of AgNPs for E. coli, S. aureus, and their mixture were 250, 125, and 500 ppm, and their MBIC-90% were 500, 250, and 1000 ppm, respectively. The estrus cycle of mice treated with co-administration of AgNPs and LC was similar to the control group (P < 0.05). The results of histology also showed that infected mice were treated with AgNPs and LC, simultaneously.
ConclusionSingle bacteria are more sensitive than their mixed model to these NPs. Co-administration of AgNPs as an antibacterial agent and LC as an antioxidant agent can treat AV in the infected mice.
Keywords: Aerobic vaginitis, Staphylococcus aureus, Escherichia coli, Metal nanoparticle, L-carnitine -
Pages 43-50Introduction
In this work, we used a thread-paper microfluidic device (μTPAD) system, where a threaded part for the handling of the whole blood samples and a paper part for the reaction of plasma with immobilized bioreagents integrated into woman pad as a wearable sensing device namely as smart women pad. The μTPAD as a wearable smart woman pad is developed for the detection of pH and urea in mensuration blood as real samples.
MethodsThis combined device was constructed to cover the elements required, that is, separation of red blood cell, conditioning, analyte reaction, and colorimetric detection. The color change in sensing areas was measured in the RGB values via a smartphone using the Color Grab after a smart woman pad was used. The thread allowed red blood cell sampling and separation, while the paper microfluidic device was used for conditioning, biorecognition, and colorimetric transduction of pH and urea as analytes.
ResultsThe time needed for analysis was measured as 110 s using the equilibrium method for both analytes, with a limit of detection (LOD) of 72.55 μg/mL for urea, with precision around 1.68%, while for pH around 0.80%. The smart woman pad allowed rapid detection of pH and urea in menstruation blood as real samples for monitoring of the kidney functions, and the results showed an agreement with the conventional methods that have been generally used in the clinical laboratory.
ConclusionThe smart woman pad has the potential to be used as a wearable device to monitor the health status of the user via its blood mensuration analysis.
Keywords: Wearable device, Smart woman pad, Thread-paper microfluidicdevice, pH, Urea, Whole blood -
Pages 51-55Introduction
Mesenchymal stromal cells (MSCs) administration is an effective option for the treatment of diabetic foot ulcers (DFUs). However, to date, studies assessing long-term outcomes and evaluating skin parameters after cell-based therapy are lacking. We presented the clinical outcomes of 3 patients, treated for DFUs with the bone marrow MSCs 3 years earlier.
MethodsUltrasound examination was used to compare collagen density and epidermal thickness in areas of healed ulcers in comparison with non-affected skin used as a control. Ultrasound and dermatoscopy were used to exclude neoplasm formation, to assess scar contracture and wound recurrence.
ResultsIn all patients, no ulcer recurrence was detected, which was lower than the expected 60% rate of re-ulceration in diabetic patients in a 3-year period (OD [odds ratio] = 0.095, P = 0.12). No neoplasm formation, no contracture of hypertrophic scar, and adjacent tissue were registered. Collagen ultrasound density was decreased by 57% (P = 0.053) and epidermal thickness was increased by 72% (P = 0.01) in the area of healed ulcers in all patients.
ConclusionMSCs therapy alone did not result in the complete restoration of the skin parameters within a 3-year period. MSCs may represent important adjuvant to the therapy, however, other novel approaches are required to achieve better results.
Keywords: Cell therapy, Diabetic foot ulcer, Mesenchymal stem cells, Mesenchymal stromal cells, Regenerative medicine, Wound healing -
Pages 57-64Introduction
Hydrogels are unique candidates for a wide range of biomedical applications including drug delivery and tissue engineering. The present investigation was designed to consider the impact of chitosan-based hydrogels as a scaffold on the proliferation of human bone marrow mesenchymal stem cells (hBM-MSCs) besides neutralization of oxidative stress in hBM-MSCs.
MethodsChitosan (CS) and CS-gelatin hydrogels were fabricated through ionic crosslinking using β-glycerophosphate. The hBM-MSCs were cultured on the prepared matrices and their proliferation was evaluated using DAPI staining and MTT assay. Furthermore, the effect of hydrogels on oxidative stress was assessed by measuring the expression of NQO1, Nrf2, and HO-1 genes using real-time PCR.
ResultsThe developed hydrogels indicated a porous structure with high water content. The toxicity studies showed that the prepared hydrogels have a high biocompatibility/cytocompatibility. The expression of intracellular antioxidant genes was studied to ensure that stress is not imposed by the scaffold on the nested cells. The results showed that Nrf2 as a super transcription factor of antioxidant genes and its downstream antioxidant gene, NQO1 were downregulated. Unexpectedly, the upregulation of HO-1 was detected in the current study.
ConclusionThe prepared CS-based hydrogels with desired properties including porous structure, high swelling ability, and cytocompatibility did not show oxidative stress for the nesting of stem cells. Therefore, they could be attractive scaffolds to support stem cells for successful tissue engineering purposes.
Keywords: Hydrogel, Chitosan, Mesenchymal stem cells, Oxidative stress, Gene expression, Tissue engineering -
Pages 65-86Introduction
Tumor endothelial marker 1 (TEM1) is expressed by tumor vascular endothelial cells in various cancers.
MethodsHere, we developed poly(lactic-co-glycolic acid) (PLGA) nanoparticles (NPs) PEGylated with polyethylene glycol (PEG) and functionalized with anti-TEM1 antibody fragment (78Fc) and loaded them with necroptosis-inducing agent shikonin (SHK) (78Fc-PLGA-SHK NPs).
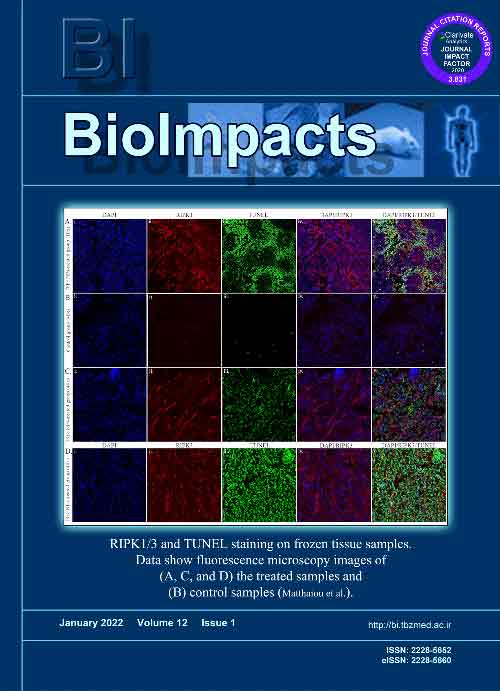
ResultsThe nanoformulation showed a smooth spherical shape (~120 nm; the ζ potential of –30 mV) with high drug entrapment and bioconjugation efficiencies (~92% and ~90%, respectively) and a sustained-release profile in serum. Having significant toxicity in vitro (e.g., MS1 and TC1 cells), the nanoformulation dramatically increased the cytotoxicity in the TC1 murine lung carcinoma subcutaneous and intravenous/metastatic models as aggressive tumor models. The injection of the 78Fc-PLGA-SHK NPs to the MS1-xenograft mice resulted in significantly higher accumulation and effects in the TEM1-positive tumor targets, while they were excreted via urine track without retaining in the liver/spleen. In the TC1 subcutaneous model, C57/BL6 mice treated with the 78Fc-PLGA-SHK NPs revealed a significant therapeutic effect. The mice, which were tumor-free after receiving the nanoformulation, were re-challenged with the TC1 cells to investigate the immune response. These animals became tumor-free a week after the injection of TC1 cells.
ConclusionBased on these findings, we propose the 78Fc-PLGA-SHK NPs as a highly effective immunostimulating nanomedicine against the TEM1-expressing cells for targeted therapy of solid tumors including ovarian cancer.
Keywords: Tumor endothelial marker 1, endosialin, CD248, Shikonin, Targeted therapy, Nanomedicine, Tumor vasculature