فهرست مطالب
Biolmpacts
Volume:13 Issue: 4, Jul 2023
- تاریخ انتشار: 1402/04/17
- تعداد عناوین: 8
-
-
Pages 269-273
Induced autoimmunity or autoinflammatory-like conditions as a rare vaccine-related adverse event have been reported following COVID-19 vaccination. Such inadvertent adverse reactions have raised somewhat concerns about the long-term safety of the developed vaccines. Such multifactorial phenomena may be related to the cross-reactivity between the viral-specific antigens with the host self-proteins through molecular mimicry mechanism and/or nonspecific bystander activation of the non-target antigen-independent immunity by the entities of the vaccine products. However, due to the low incidence of the reported/identified individuals and insufficient evidence, autoimmunity following the COVID-19 vaccination has not been approved. Thereby, it seems that further designated studies might warrant post-monitoring of the inevitable adverse immunologic reactions in the vaccinated individuals, especially among hypersensitive cases, to address possible immunological mechanisms induced by the viral vaccines, incorporated adjuvants, and even vaccine delivery systems.
Keywords: Autoimmunity, Bystander activation, COVID-19 vaccine, Molecular mimicry, Post-vaccination -
Pages 275-287Introduction
Recently, the application of nanofibrous mats for dressing skin wounds has received great attention. In this study, we aimed to fabricate and characterize an electrospun nanofibrous mat containing polycaprolactone (PCL), chitosan (CTS), and propolis for use as a tissue-engineered skin substitute.
MethodsRaw propolis was extracted, and its phenolic and flavonoid contents were measured. The physiochemical and biological properties of the fabricated mats, including PCL, PCL/CTS, and PCL/CTS/Propolis were evaluated by scanning electron microscopy (SEM), atomic force microscopy (AFM), mechanical analysis, swelling and degradation behaviors, contact angle measurement, cell attachment, DAPI staining, and MTT assay. On the other hand, the drug release pattern of propolis from the PCL/CTS/Propolis scaffold was determined. A deep seconddegree burn wound model was induced in rats to investigate wound healing using macroscopical and histopathological evaluations.
ResultsThe results revealed that the propolis extract contained high amounts of phenolic and flavonoid compounds. The fabricated scaffold had suitable physicochemical and mechanical properties. Uniform, bead-free, and well-branched fibers were observed in SEM images of mats. AFM analysis indicated that the addition of CTS and propolis to PCL elevated the surface roughness. MTT results revealed that the electrospun PCL/CTS/Propolis mat was biocompatible. The presence of fibroblast cells on the PCL/CTS/Propolis mats was confirmed by DAPI staining and SEM images. Also, propolis was sustainably released from the PCL/CTS/Propolis mat. The animal study revealed that addition of propolis significantly improved wound healing.
ConclusionThe nanofibrous PCL/CTS/Propolis mat can be applied as a tissue-engineered skin substitute for healing cutaneous wounds, such as burn wounds.
Keywords: Skin substitutes, Polycaprolactone, Chitosan, Propolis, Nanofibers, Wound healing -
Pages 289-300Introduction
Pluripotent stem cells have been used by various researchers to differentiate and characterize endothelial cells (ECs) and vascular smooth muscle cells (VSMCs) for the clinical treatment of vascular injuries. Studies continue to differentiate and characterize the cells with higher vascularization potential and low risk of malignant transformation to the recipient. Unlike previous studies, this research aimed to differentiate induced pluripotent stem (iPS) cells into endothelial progenitor cells (EPCs) and VSMCs using a step-wise technique. This was achieved by elucidating the spatio-temporal expressions of the stage-specific genes and proteins during the differentiation process. The presence of highly expressed oncogenes in iPS cells was also investigated during the differentiation period.
MethodsInduced PS cells were differentiated into lateral mesoderm cells (Flk1+). The Flk1+ populations were isolated on day 5.5 of the mesodermal differentiation period. Flk1+ cells were further differentiated into EPCs and VSMCs using VEGF165 and platelet-derived growth factor-BB (PDGFBB), respectively, and then characterized using gene expression levels, immunocytochemistry (ICC), and western blot (WB) methods. During the differentiation steps, the expression levels of the marker genes and proto-oncogenic Myc and Klf4 genes were simultaneously studied.
ResultsThe optimal time for the isolation of Flk1+ cells was on day 5.5. EPCs and VSMCs were differentiated from Flk1+ cells and characterized with EPC-specific markers, including Kdr, Pecam1, CD133, Cdh5, Efnb2, Vcam1; and VSMC-specific markers, including Acta2, Cnn1, Des, and Myh11. Differentiated cells were validated based on their temporal gene expressions, protein synthesis, and localization at certain time points. Significant decreases in Myc and Klf4 gene expression levels were observed during the EPCs and VSMC differentiation period.
ConclusionEPCs and VSMCs were successfully differentiated from iPS cells and characterized by gene expression levels, ICC, and WB. We observed significant decreases in oncogene expression levels in the differentiated EPCs and VSMCs. In terms of safety, the described methodology provided a better safety margin. EPCs and VSMC obtained using this method may be good candidates for transplantation and vascular regeneration.
Keywords: Induced pluripotent stem cells, Endothelial progenitor cells, Vascular smooth muscle cells, Lateral mesoderm cell, In vitro differentiation -
Pages 301-311Introduction
Silymarin proved to be a beneficial herbal medicine against many hepatic disorders such as alcoholic liver disease (ALD). However, its application is restricted due to its low bioavailability and consequently decreased efficacy. We herein used a nano-based approach known as “phytosome”, to improve silymarin bioavailability and increase its efficacy.
MethodsPhytosome nanoparticles (NPs) were synthesized using thin film hydration method. NPs size, electrical charge, morphology, stability, molecular interaction, entrapment efficiency (EE %) and loading capacity (LC %) were determined. Moreover, in vitro toxicity of NPs was investigated on mesenchymal stem cells (MSCs) viability using MTT assay. In vivo experiments were performed using 24 adult rats that were divided into four groups including control, ethanol (EtOH) treatment, silymarin/EtOH treatment and silymarin phytosome/EtOH, with 6 mice in each group. Experimental groups were given 40% EtOH, silymarin (50 mg/kg) and silymarin phytosome (200 mg/kg) through the gastric gavage once a day for 3 weeks. Biochemical parameters, containing ALP, ALT, AST, GGT, GPx and MDA were measured before and after experiment to investigate the protective effect of silymarin and its phytosomal form. And histopathological examination was done to evaluate pathological changes.
ResultsSilymarin phytosome NPs with the mean size of 100 nm were produced and were well tolerated in cell culture. These NPs showed a considerable protective effect against ALD through inverting the biochemical parameters (ALP, ALT, AST, GGT, GPx) and histopathological alterations
ConclusionSilymarin phytosomal NPs can be used as an efficient treatment for ALD.
Keywords: Silymarin, Phytosome, Nanoparticles, Alcoholic liver disease -
Pages 313-321Introduction
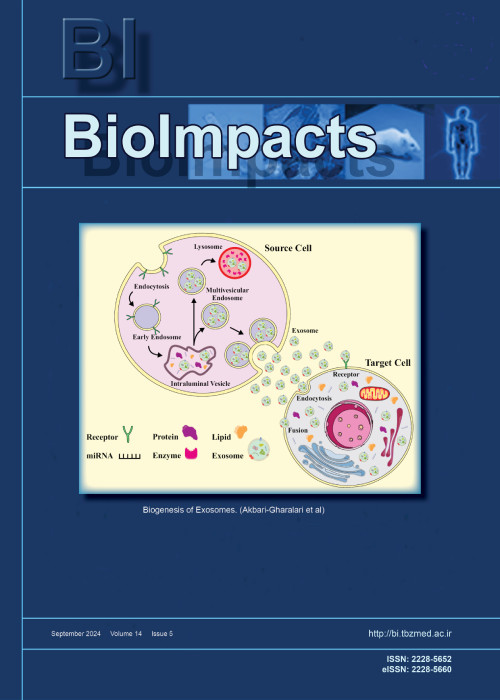
Resistance to chemotherapy and/ or irradiation remains one of the key features of malignant tumors, which largely limits the efficiency of antitumor therapy. In this work, we studied the progression mechanism of breast cancer cell resistance to target drugs, including mTOR blockers, and in particular, we studied the exosome function in intercellular resistance transfer.
MethodsThe cell viability was assessed by the MTT assay, exosomes were purified by successive centrifugations, immunoblotting was used to evaluate protein expression, AP-1 activity was analyzed using reporter assay.
ResultsIn experiments on the MCF-7 cell line (breast cancer) and the MCF-7/Rap subline that is resistant to rapamycin, the capability of resistant cell exosomes to trigger a similar rapamycin resistance in the parent MCF-7 cells was demonstrated. Exosome-induced resistance reproduces the changes revealed in MCF-7/Rap resistant cells, including the activation of ERK/AP-1 signaling, and it remains for a long time, for at least several months, after exosome withdrawal. We have shown that both the MCF-7 subline resistant to rapamycin and cells having exosome-triggered resistance demonstrate a stable decrease in the expression of DNMT3A, the key enzyme responsible for DNA methylation. Knockdown of DNMT3A in MCF-7 cells by siRNA leads to partial cell resistance to rapamycin; thus, the DNMT3A suppression is regarded as one of the necessary elements for the development of acquired rapamycin resistance.
ConclusionWe propose that DNA demethylation followed by increased expression of key genes may be one of the factors responsible for the progression and maintenance of the resistant cell phenotype that includes exosome-induced resistance.
Keywords: Breast cancer, DNMT3A, Signaling, Rapamycin resistance, Exosomes -
Pages 323-332Introduction
Computational modeling is one of the best non-invasive approaches to predicting the functional behavior of the mitral valve (MV) in health and disease. Mitral valve prolapse (MVP) due to partial or complete chordae tendineae rapture is the most common valvular disease and results in mitral regurgitation (MR).
MethodsIn this study, Image-based fluid-structure interaction (FSI) models of the human MV are developed in the normal physiological and posterior leaflet prolapse conditions. Detailed geometry of the healthy human MV is derived from Computed Tomography imaging data. To provide prolapse condition, some chords attached to the posterior leaflet are removed from the healthy valve. Both normal and prolapsed valves are embedded separately in a straight tubular blood volume and simulated under physiological systolic pressure loads. The Arbitrary Lagrangian-Eulerian finite element method is used to accommodate the deforming intersection boundaries of the blood and MV.
ResultsThe stress values in the mitral components, and also flow patterns including the regurgitant flow rates are obtained and compared in both conditions through the simulation. These simulations have the potential to improve the treatment of patients with MVP, and also help surgeons to have more realistic insight into the dynamics of the MV in health and prolapse.
ConclusionIn the prolapse model, computational results show incomplete leaflet coaptation, higher MR severity, and also a significant increment of posterior leaflet stress compared to the normal valve. Moreover, it is found more deviation of the regurgitant jet towards the left atrium wall due to the posterior leaflet prolapse.
Keywords: Fluid-structure interaction, Human mitral valve, Mitral valve prolapse, Computed tomographyimaging, Hemodynamics, Chordae tendineae rupture -
Pages 333-346Introduction
The maturation faith of dendritic cells is restrained by the inflammatory environment and cytokines, such as interleukin-6 and its downstream component. Therefore, introducing the suitable antigen to dendritic cells is crucial. However, reducing the severity of the suppressive tumor microenvironment is indispensable. The present study examined the combination therapy of lymphocyte antigen 6 family member E (LY6E) pulsed mature dendritic cells (LPMDCs) and pioglitazone against colorectal cancer (CRC) to elevate the effectiveness of cancer treatment through probable role of pioglitazone on inhibiting IL-6/STAT3 pathway.
MethodsDendritic cells were generated from murine bone marrow and were pulsed with lymphocyte antigen 6 family member E peptide to assess antigen-specific T-cell proliferation and cytotoxicity assay with Annexin/PI. The effect of pioglitazone on interleukin (IL)-6/STAT3 was evaluated in vitro by real-time polymerase chain reaction (PCR). Afterward, the CRC model was established by subcutaneous injection of CT26, mouse colon carcinoma cell line, in female mice. After treatment, tumor, spleen, and lymph nodes samples were removed for histopathological, ELISA, and real-time PCR analysis.
ResultsIn vitro results revealed the potential of lysate-pulsed dendritic cells in the proliferation of double-positive CD3-8 splenocytes and inducing immunogenic cell death responses, whereas pioglitazone declined the expression of IL-6/STAT3 in colorectal cell lines. In animal models, the recipient of LPMDCs combined with pioglitazone demonstrated high tumor-infiltrating lymphocytes. Elevating the IL-12 and interferon-gamma (IFN-γ) levels and prolonged survival in lysate-pulsed dendritic cell and combination groups were observed.
ConclusionPioglitazone could efficiently ameliorate the immunosuppressive feature of the tumor microenvironment, mainly through IL-6. Accordingly, applying this drug combined with LPMDCs provoked substantial CD8 positive responses in tumor-challenged animal models.
Keywords: Tumor-associated antigen, Dendritic cells, Thiazolidinediones, Colorectal cancer, Lymphocyte antigen 6 familymember E, Pioglitazone -
Pages 347-353Introduction
In this work, a flexible, and wearable point-of-care (POC) device integrated on a pain relief patch as wearable colorimetric sensors have been developed for sweat analysis, such as lactic acid, sodium ions, and pH simultaneously. Herein, the patch has still functioned as pain relief, while it allows for sweat monitoring during exercise, and in daily activities.
MethodsIt was constructed on cotton cloth using wax printing technology (batik stamp) as cloth-based microfluidic devices (CMDs). Here, it uses micro volumes of samples to perform the reaction in the sensing zones, where the sensitive reagents are immobilized so that it can collect and analyze the sweat (lactic acid, sodium ions, and pH) as the model for sweat analytes. The colorimetric analysis was conducted via a smartphone camera by using a free app (Color Grab) for a color image analysis that uses for quantitative analysis or naked eye for semi-qualitative analysis.
ResultsThe ΔRGB value of the CMDS shows the excellent linear correlation vs analytes concentration, where the coefficient of correlations was found for lactic acid (R2 = 0.994), sodium ion (R2 = 0.998), and pH (R2 = 0.994). The ΔRGB value shows the appropriate color value for the linear correlation of the analyte target concentrations in the sweat samples. Here, the limit of detection (LOD) was found at 45.73 μg/mL for lactic acid and 56.46 μg/mL for sodium ions. The reproducibility was found at 0.79% and 0.89%, for lactic acid and sodium ions respectively.
ConclusionIt was applied for sweat analysis during exercise, and the results show in agreement with the standard methods used in a clinical laboratory.
Keywords: Wearable device, Smart patch, Cloth-basedmicrofluidic devices, Sweat analysis